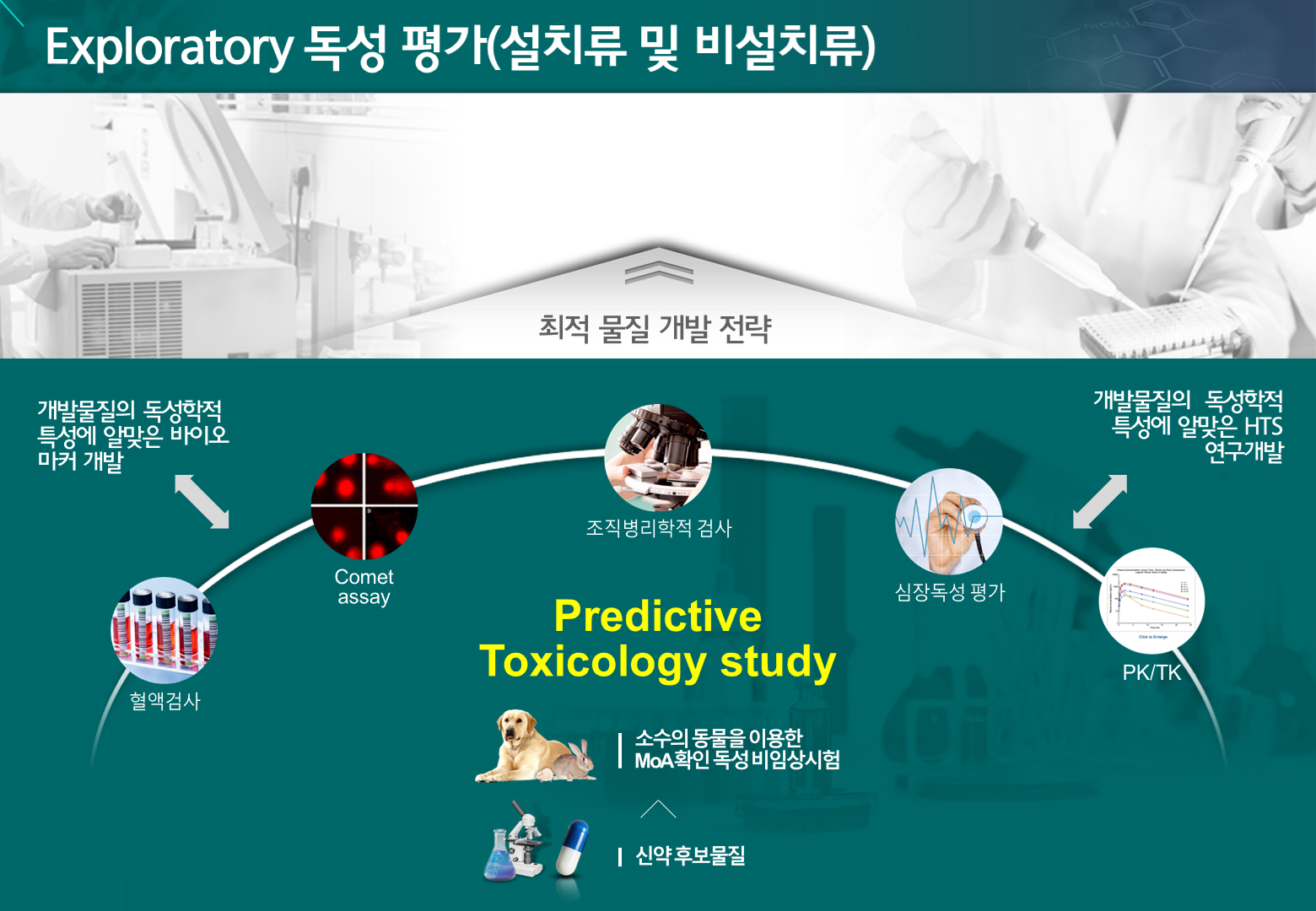
The Non-clinical Development Center provides services to accurately identify non-clinical safety issues related to substances under development. When necessary, we conduct hypothesis-driven experiments and offer solutions through comprehensive data analysis conducted by experts from various disciplines.

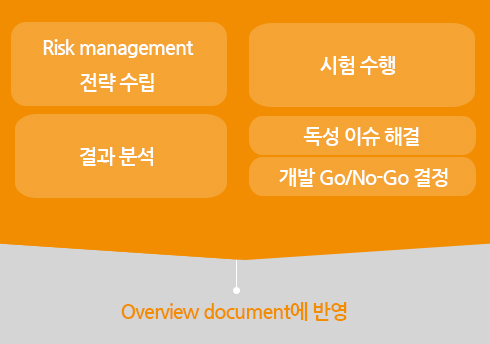
Strategic Planning for Risk Management
Execution of Experiments
Data Analysis
Toxicity Issue Solution
Development Go/No-Go Decision
Reflected in the Overview Document
Type 1
Mechanism
Identification Test
Knowledge-Based Approach.
If non-clinical safety issues can be explained and resolved through various literature sources and past cases, the results are presented as an expert report.
Type 2
Solution Derived from Existing Knowledge
(Experience, Knowledge, Literature Review, etc.)
Conducting Additional Testing
If additional assays are required to accurately identify non-clinical safety issues, various assays are conducted to seek a resolution.
Type 3
Hypothesis-driven Study for MoA
Establishing a new hypothesis and designing appropriate tests.
Conducting hypothesis-driven tests to identify and resolve non-clinical safety issues.
List of Available Services.
에
새로운 글이 작성되었습니다.